Abstract
Introduction
Sickle cell disease (SCD) is caused by pathologic variants in both alleles of the β-globin gene, affecting ~100,000 patients in the US (Strouse. Handb Clin Neurol 2016;138:311-24). Elevated fetal hemoglobin (HbF) levels ameliorate symptoms and improve survival in patients with SCD (Hebert. Am J Hematol 2020;95:1235-45). SAR445136 (BIVV003) is a novel therapeutic product comprising autologous CD34+ HSPCs modified ex vivo by zinc finger nucleases (ZFN) and targeting the BCL11A gene erythroid-specific enhancer (ESE) to increase endogenous HbF production.
Methods
PRECIZN-1 (NCT03653247) is an ongoing first-in-human, open label, single arm, multi-site study evaluating safety and tolerability of SAR445136 (n=8; aged 18-40 years), with severe SCD across 6 US sites. Eligible subjects underwent mobilization and apheresis with plerixafor 240 ug/kg/day for up to 3 days to collect autologous CD34+ HSPCs to achieve a minimum of 10 × 10 6 CD34+ HSPC/kg for manufacturing of the SAR445136 dose. Additional apheresis cycles were allowed to achieve the minimum cell dose and rescue aliquots. Autologous HSPCs were transfected ex vivo with ZFN mRNAs targeting the ESE region of the BCL11A locus to manufacture SAR445136. A single IV infusion of 3-20 × 10 6 CD34+ HSPC/kg was administered at least 72 hours after the final busulfan myeloablation dose. Subjects were monitored for stem cell engraftment and hematopoietic recovery, adverse events (AEs), clinical and laboratory hemolysis markers, total Hb and HbF, percentage of F cells and sickle-cell related events post-SAR445136 infusion. Health-related quality of life (HRQoL) was assessed via the PROMIS-57 survey at screening, Weeks 26 and 52, and early termination visit.
Results
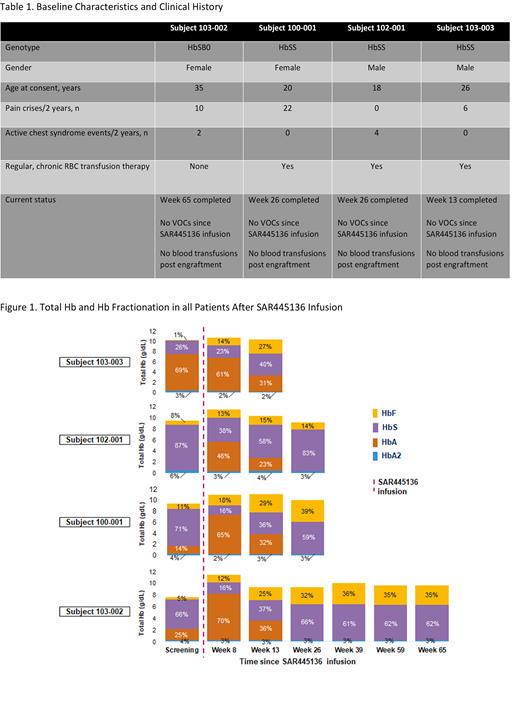
Of the 7 subjects that underwent mobilization and apheresis to date (25 June 2021), 5 achieved successful target yields ranging from 3.4-13.8 x 10 6 CD34+ HSPC/kg per apheresis day (mean: 6.49 x10 6 CD34+ HSPC/kg per apheresis day) in one apheresis cycle (4.45-10.9 x 10 6 CD34+ HSPC/kg per 2-day cycle). One subject failed to mobilize; one discontinued due to intercurrent cholangitis. Baseline patient characteristics of the 4 patients infused are in Table 1.
Pre-apheresis peripheral blood WBC ranged from 23.2-36.9 x 10 3/μL (mean: 28.7 x 10 3/μL) and % CD34+ was 0.09-0.36% (0.22%) with absolute CD34+ counts of 20-80/μL (mean: 60/μL). Four of the mobilized subjects were successfully infused with SAR445136 at a single dose ranging from 3.2-9.7 x 10 6 CD34+ HSPC/kg (mean: 5.17 x 10 6 CD34+ HSPC/kg). All 4 subjects engrafted with a median time to neutrophil and platelet recovery of 21.5 and 24.5 days, respectively. No rescue doses were required.
All 4 patients improved clinically since SAR445136 infusion, with no recurrence of previous vaso-occlusive crises (VOCs). Total Hb stabilized at 9-10 g/dL by week 26 post SAR445136 infusion along with improvements in the clinical markers of hemolysis in all 4 subjects. Percent HbF levels were 1-11% at screening, increasing to 15-29% by Week 13 in all 4 subjects, to 14-39% by Week 26 in the 3 subjects with at least 26 weeks follow up; and persisting at 35% in 1 subject with 65 weeks follow up (Figure 1). Percent F cells increased to 49-94% in 3 subjects with at least 26 weeks follow up, persisting at 90% in 1 subject with 65 weeks follow up. The fourth subject had 87.5% F cells at 13 weeks follow up.
Although preliminary, a trend of improvement exceeding the proposed minimally clinically important difference in all PROMIS-57 HRQoL domains except sleep disturbance was observed in the 3 subjects with 26 weeks follow up, whose scores were "worse" than the norm at baseline (≤2 points per domain).
SAR445136 was generally well tolerated with no infusion related reactions. The AEs reported were consistent with plerixafor mobilization and busulfan myeloablation therapy. No AEs or SAEs were reported as related to SAR445136.
Conclusions
As of 25 June 2021, these preliminary proof-of-concept efficacy and safety results confirm the potential therapeutic value of ZFN-mediated modification of the BCL11A ESE region and SAR445136 infusion to address current unmet needs of patients with SCD. All 4 infused patients had no SCD related events including VOCs following SAR445136 infusion, as well as increases in total Hb, HbF, and %F cells, and clinical improvements in PROMIS-57 domains. SAR445136 is generally well tolerated in the 4 subjects infused to date, with no related AEs or SAEs reported.
Abedi: BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Speakers Bureau; Abbvie: Speakers Bureau. Galeon: Sanofi: Current Employment. Reiner: Sanofi: Current Employment. Smith: Sanofi: Current Employment. Wang: Sanofi: Current Employment. Ramezi: Sanofi: Current Employment. Rendo: Sanofi: Current Employment, Other: May hold shares and/or stock options . Walters: AllCells, Inc: Consultancy; Vertex pharmaceuticals: Consultancy; Ensoma, Inc.: Consultancy; BioLabs, Inc: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal